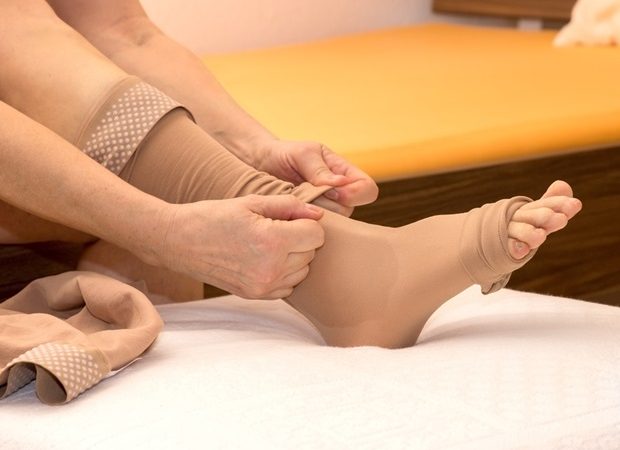
Octapharma USA today announced the U.S. Food & Drug Administration (FDA) has approved cutaquig® [Immune globulin, Subcutaneous (Human)-hipp, 16.5% Solution] for the treatment of pediatric patients age 2 and older with primary humoral immunodeficiency (PI). The FDA previously approved cutaquig® for adults with PI.
“The FDA approval provides physicians and families with more treatment options for patients with primary immune disorders, which weaken the immune system and can allow infections and other health issues to occur more easily,” said Roger H. Kobayashi, M.D., Clinical Professor UCLA School of Medicine and National Consultant, Immune Deficiency Foundation. “The FDA approval also provides more flexible options by permitting more frequent or less frequent infusions, which can be advantageous based on a patient’s pharmacokinetic and clinical response.”
Patients and providers have the flexibility to administer cutaquig® at a lower dose more frequently or at a larger dose less frequently if desired. Patients who prefer less frequent injections may have the option of receiving therapy every other week. At the same time, physicians can prescribe daily dosing if patients respond better to more frequent therapy.
Cutaquig® provides enhanced convenience for a wider group of patients who want to customize therapy with their prescriber to best match patient lifestyle needs,” said Octapharma USA President Flemming Nielsen. “Octapharma is committed to providing people with immune disorders the life-saving therapies they need. Both the addition of the pediatric indication and the flexible dosing illustrate our commitment to ensure patients have access to lifesaving products that offer a variety of choices for therapy delivery.”
Flemming Nielsen, President, Octapharma USA
The FDA approval of cutaquig® is based on the results of two clinical trials, which observed 75 PI patients, 37 adults and 38 pediatric patients between ages 2 and 17. The patients received weekly infusions with cutaquig® during a 12-week wash-in/wash-out period followed by a 12-month efficacy period. The main objective of the research was to assess the efficacy of cutaquig® in preventing serious bacterial infections, defined as bacteremia/sepsis, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia and visceral abscess. No serious bacterial infections were reported.